|
Acute Aortic Dissection - Quick Consult |
|
|
|
|
|
Last Updated / Reviewed: June 2022
|
|
Definitions
Pathophysiology
Key History
Key Physical Exam
Risk Factors for AAD / AoD
Classification Systems
Differential Diagnosis
Laboratory & Imaging
|
Acute Aortic Syndromes:
- Aortic Dissection (AoD). This includes Acute Aortic Dissection (TAD), and the
abbreviations may be used interchangeably. AoD is defined as disruption of the media
layer of the aorta with bleeding within and along the wall of the aorta resulting in
separation of the layers of the aorta. In the majority of patients (90%), an intimal
disruption is present that results in tracking of the blood in a dissection plane
within the media.
- Intramural Hematoma (IMH). When the term IMH is used strictly, there is no
intimal defect such as a tear or an ulcer. On noninvasive imaging, 15% of patients
with aortic dissection syndromes have an apparent IMH without evidence of an intimal
tear. Autopsy studies show only 4% have no visible intimal tear; indeed, at the time
of surgery, a tear is found in most patients.
- Penetrating Atherosclerotic Ulcer (PAU). Extensive atheromatous disease of
the aorta may lead to PAU or a localized IMH. PAU may then lead to more severe
aortic disease, including IMH and AoD.
Timing of Onset of Initial Symptoms to Presentation
- Acute dissection is defined as occurring within 2 weeks of onset of pain.
- Subacute is defined as occurring between 2 and 6 weeks from onset of pain.
- Chronic is defined as occurring more than 6 weeks from onset of pain.
Thoracoabdominal Aneurysm (TAA): Aneurysm involving the acute and abdominal aorta
Abdominal Aortic Aneurysm (AAA): Aneurysm involving the infradiaphragmatic
abdominal aorta
|
|
Back To Top
|
|
The aorta, like most other arteries, consists of three layers: the intima, the media,
and the adventitia. Aortic dissection involves separation of the intima from the
adventitia within the aortic wall by a dissecting column of blood propagating through
the media via longitudinal cleavage.
Once the dissecting hematoma is established in the media of the aorta, migration
can occur either in an antegrade or retrograde fashion, creating a false lumen within
the outer half of the media.
The dissection progresses until it ruptures, either back into the true lumen resulting
in a "double barrel" aorta and a rare "spontaneous cure," or more commonly, the
dissection may rupture out of the adventitia into the pericardium or pleural cavity.
The majority of deaths from aortic dissection are due to rupture of the aorta into the
pericardium with subsequent death due to tamponade, or rupture into the pleural cavity
with death due to exsanguination.
The severity of the dissection is proportional to the blood pressure and the velocity
of ventricular contraction. This fact is utilized in the initial medical treatment
of aortic dissection.
The most common site of dissection is the first few centimeters of the ascending
aorta, with 90% occurring within 10 centimeters of the aortic valve. The second
most common site is just distal to the left subclavian artery. Between 5% and 10%
of dissections do not have an obvious intimal tear.
Location: 65% in ascending aorta; 10% in aortic arch; 25% in descending aorta (upper
portion)
|
|
Back To Top
|
- Sudden or abrupt onset of pain
- Ripping or tearing pain in the intrascapular area
- Key: Movement or migration of pain from chest or upper back to the lower
back or
abdomen
|
- Acute, severe chest pain (anterior chest pain can mimic acute myocardial
infarction)
- Pain extending to the neck or jaw
- Altered mental status
|
|
|
Back To Top
|
|
|
- Diplopia
- Dysphagia
- Dyspnea
- Flank pain if the renal arteries are involved
- Abdominal findings related to mesenteric ischemia
- Horner’s syndrome
|
- Hypertension
- Hypotension if associated with
cardiac tamponade, hypovolemia, excessive vagal tone
- Limb paresthesias
- Pulse deficit carotid, brachial or femoral pulse (i.e., weak or no
pulse)
- A difference of 20 mm Hg between the arms
- Syncope
|
|
|
Back To Top
|
|
|
Conditions Associated With Increased Aortic Wall Stress
Hypertension, particularly if uncontrolled
Pheochromocytoma
Cocaine or other stimulant use
Weightlifting or other Valsalva maneuver
Trauma
Deceleration or torsional injury (e.g., motor vehicle crash, fall)
Coarctation of the aorta
Conditions Associated With Aortic Media Abnormalities
-
Genetic
Marfan syndrome
Ehlers-Danlos syndrome, vascular form
Bicuspid aortic valve (including prior aortic valve replacement)
Turner syndrome
Loeys-Dietz syndrome
Familial acute aortic aneurysm and dissection syndrome
-
Inflammatory Vasculitides
Takayasu arteritis
Giant cell arteritis
Behçet arteritis
-
Other
Pregnancy
Polycystic kidney disease
Chronic corticosteroid or immunosuppression agent administration
Infections involving the aortic wall either from bacteremia or extension of
adjacent infection
|
|
Back To Top
|
|
|
|
|
Back To Top
|
|
|
|
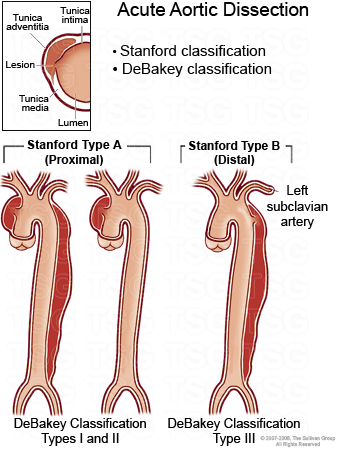
DeBakey Classification
The DeBakey classification divides aortic dissection into three types:
|
|
Type I |
Dissections begin in the ascending aorta and extend distally to involve the
aortic
arch and the descending aorta. |
|
Type II |
Dissections involve only the ascending aorta. |
|
Type III |
Dissections involve the descending aorta, distal to the left subclavian
artery.
Dissections may also propagate in a retrograde fashion to involve the
proximal aorta.
|
|
Type IIIA |
Dissections stop above the diaphragm. |
|
Type IIIB |
Dissections propagate below the diaphragm. |
|
|
Stanford Classification
In the Stanford classification, all dissections involving the ascending
aorta are Type A.
All other dissections are Type B.ii
|
|
Type A |
Any dissection involving the proximal aorta. Corresponds to DeBakey Types I and
II. Accounts for about 70% of cases. |
|
Type B |
Dissection of the distal aorta. Corresponds to DeBakey Type III. Accounts for
about
30% of cases.
|
|
|
Type A = Surgical emergency w/mortality 15%-20%; if medically treated, 70%-80%;
type B managed medically, 11%
|
|
Back To Top
|
|
|
- Acute sickle cell
chest syndrome
- Cholecystitis
- Anxiety disorder
- Aortic stenosis
- Cholelithiasis
- Congestive heart failure
- Coronary artery disease
- Costochondritis
- Esophageal rupture
- Esophagitis
- Gastritis
- GERD
- Herpes zoster
- Hiatal hernia
- Hypertrophic cardiomyopathy
- Kawasaki syndrome
- Endocarditis
|
|
|
|
Back To Top
|
|
|
- Choice of imaging test depends on clinical setting and availability.
- CT angiogram (CTA) is most commonly used.
- Different protocol than CTPA (pulmonary artery) that is used to
rule out pulmonary embolism (PE).
- If both PE and TAD are in the differential diagnosis, order CTA
to rule out TAD and also PE. The dissection protocol is
excellent at detecting PE, but it is always helpful to alert the
radiologist that you are concerned about both.
- If a high clinical suspicion exists for TAD but initial aortic imaging
is negative, a second imaging study should be obtained.
- Transthoracic echocardiography (TTE) is not sensitive enough to exclude
TAD from the differential diagnosis; however, some dissections are seen
on TTE—in those instances, TTE is diagnostic.
- ECG and cardiac markers are usually normal; D-dimer is sensitive but
nonspecific.
- Chest X-ray is abnormal in 80%-90% and may include: depression of left
main stem bronchus, loss of aortic-pulmonary window, left sided
effusion, apical cap, obliteration of aortic knob, or trachea deviated
to right.
|
|
|
Back To Top
|
|
|
